Linguistic diversity in the UK
It’s a commonly held belief that the British are not language learners. More than 9 in 10 Brits speak only English and it’s certainly true that, in a world largely dominated by English-language communications, we aren’t driven to learn languages with the same urgency as other European countries. We’ll see in this article that demand for translations for UK clinical trials, suggests much linguistic greater diversity than one might assume.
One might thus imagine that we are a nation entirely made up of English speakers. Indeed, when compared with Scandinavian countries, where more than half of the population is fluent in English as a second language, we may well appear to be relatively monolingual.
At present, 4.2 million people in England and Wales speak a first language other than English, and of those, around 730,000 say that they cannot speak English well. Among those for whom English is not a first language, around 140,000 residents do not speak the language at all.
Despite the relatively low number of us with a second or third language, London alone is home to speakers of as many as 300 other languages.
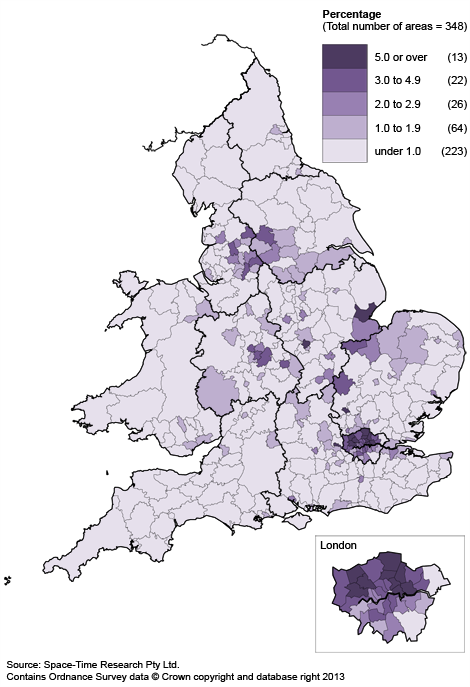
Of course, minority languages vary according to Local Area Authority. For example, according to the last census in 2011, Bengali is officially the most commonly spoken language in London after English, whereas Slough had highest proportion of speakers of Punjabi (6.2%) as their main language. Leicester had the highest proportion of Gujarati speakers (11.5%) and Boston in Lincolnshire reported the highest proportion of Lithuanian speakers (2.8%).
The last census across England and Wales in 2011 revealed that Polish was the most widely spoken language after English, but Turkish, Gujarati, Punjabi, Urdu, Arabic and Tamil are all widely spoken in many of our cities.
We all benefit culturally from the rich tapestry of languages spoken among our families and communities, as well as high-streets and religious institutions.
And it is frequently native speakers of these languages for whom patient information must be translated and presented in an accessible, comprehensible manner. This is especially true for clinical trials.
As companies in the UK scramble to conduct meaningful COVID-19 trials, it is frequently in highly populated areas where we see ‘spikes’ in infection and a demand by study sites for participation in trials.
This situation was described to me by an authority in UK Clinical Trials as a giant game of ‘whack-a-mole’, and that every effort was being made to set up, recruit participants and conduct trials before each spike in cases abated.
In areas where ethnic minority groups are hit by the virus, it is essential to not only understand which languages patient materials need to be translated into, but also to source these services quickly and efficiently.
With our customers working to conduct meaningful clinical trials for therapies and vaccines to tackle COVID-19, we are seeing a ‘spike’ of our own in the types of translations they need. Most notably, we are noticing a higher demand for Urdu, Punjabi, Romanian and Polish translations due to the many trials being conducted in our cities.
Our customers who are running COVID-19 trials in areas like Manchester (Britain’s ‘city of languages’) and Leicester (where cases of the virus spiked in late June of this year) all require fast translation turnarounds in time to secure consent from and recruit patients as quickly as possible.
An awareness of these ever-evolving language requirements is important when considering what translations you will need for clinical trials in the UK.
Essential documents to translate for non-English speakers in the UK include:
- Informed Consent Form (ICF)
- Patient Information Sheet (PIS)
- Questionnaires
Other clinical trial documents we can handle:
- Clinical study protocols
- Protocol synopses
- Patient/Doctor information sheets
- Investigator’s Brochures (IBs)
- Trialists’ CVs
- Ethics Committee letters
- Case Report Forms
- Adverse Event Report (AER) forms
- Insert and label text/labelling
- Appointment reminders and follow-up cards
- Patient Reported Outcomes (PROs)
- Quality of Life (QoL) instruments
- Patient diaries and questionnaires
- Interim or final reports
We are committed to providing the highest-standard translations to the life sciences industry. For the solutions we offer for clinical trial translations. Click here to contact us for a quotation.